 Summary
Summary
Oxygen isotope composition of leaf water and plant organic material
Author
Margaret Barbour
Definition
After 16O (99.7%), 18O is the next most abundant stable isotope of oxygen (0.2%). 17O also exists in nature, but at such low abundances that measurement techniques are very challenging. In plant ecological physiology the stable oxygen isotope composition refers to the relative abundance of 18O to 16O.
The oxygen isotope composition of leaf water is determined by the composition of plant water sources and leaf evaporative conditions. Leaf water is usually enriched in the heavy oxygen isotope, 18O, and this enrichment is passed on to organic molecules via isotopic exchange between water and oxygen atoms in carbonyl groups. Hence, the oxygen isotope composition of plant water and organic material has been shown to be a valuable record of leaf evaporative processes, including stomatal conductance.
Dual isotope approaches can provide additional information on environmental and plant physiological processes. For example, combined 13C and 18O analysis of plant tissue is useful for determining the processes driving changes in plant transpiration efficiency (see link to 13C carbon isotopes), while combining deuterium and 18O measurements provides information on evaporative processes (see link to hydrogen/deuterium ratios ).
Terminology and equations
The oxygen isotope composition of a sample can be expressed relative to the Vienna Standard Mean Oceanic Water standard (VSMOW), in parts per mil (‰ i.e. parts per thousand):
 where
where
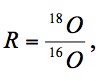
or as the difference between the substrate and the product:
 .
.
Over the years a number of theoretical models have been developed to describe processes determining 18O of leaf water and plant organic material. At isotopic steady state the degree of evaporative enrichment of leaf water above soil water ( 18Oes) is given by (Craig and Gordon 1965; Dongmann et al. 1974; Farquhar and Lloyd 1993):
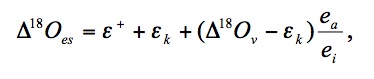
where + is the proportional depression of water vapourby the heavier H218O, k is kinetic fractionation during diffusion through the stomata and leaf boundary layer, 18Ov is the oxygen isotope composition of water vapour relative to source water and ea/ei is the ratio of ambient to intercellular vapour pressure.

where Tl is leaf temperature in K (Bottinga and Craig 1969).

where gs and gb are stomatal and leaf boundary layer conductances, respectively.
The above equations predict broad trends in bulk leaf water enrichment well, but can both over- and under-estimate enrichment due to the presence of pools of leaf water (i.e. in vascular tissue) that are not exposed to evaporative enrichment (e.g. Yakir et al. 1990; Roden et al. 2000), strings of inter-connected leaf water pools with differing enrichments (e.g. Helliker and Ehleringer 2000), diurnal changes in water content (e.g. Cernusak et al. 2002), and backward diffusion of enrichment from the evaporative sites in opposition to convection of unenriched water via the transpiration stream (e.g. Farquhar and Lloyd 1993), or a combination of these processes (e.g. Cuntz et al. 2007; Ogée et al. 2007). The reader is encouraged to read the papers cited above for further details.
Leaf evaporative enrichment is passed onto organic molecules due to isotopic exchange between carbonyl oxygen and water, with an equilibrium fractionation ( WC) resulting in between 25 and 30‰ more enriched organic oxygen (Sternberg and DeNiro 1983). Cernusak et al. (2003) found that sucrose was 27‰ more enriched than lamina water. The oxygen isotope enrichment of cellulose and whole leaf tissue above source water ( 18Oc and 18Op, respectively) are given by (Barbour and Farquhar 2000):
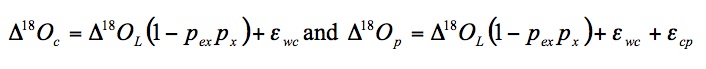
where 18OL is the oxygen isotope composition of lamina water relative to source water, pex is the proportion of exchangeable oxygen in cellulose formed from simple carbohydrates, px is the proportion of unenriched (source) water in the developing cells and ( cp) is an empirical term describing the difference in 18O between cellulose and whole leaf tissue.
Measurement approaches
18Oc and 18Op are routinely measured on an isotope ratio mass spectrometer, and a number of laboratories worldwide are able to provide this service for a fee (labs listed below). 18O of liquid samples are also measured on IRMS, usually via an equilibration technique with CO2. However, there also newly-developed laser instruments available for liquid and vapour isotope analysis. Water is usually extracted from plant tissue and soil prior to analysis on either and IRMS or a laser. Care must be taken with sampling and storage to avoid evaporative enrichment effects prior to analysis. Common water extraction techniques are aezotropic distillation and cryogenic extraction. Bulk leaf tissue can be used for 18Op analysis, but care must be taken if samples are high in nitrogen. Alternatively, samples can be purified prior to analysis (for example, sucrose or cellulose (see associated pages: TNC Total Non-structural Carbohydrates and Structural compounds ).
Laboratories providing 18O analytical services:
- Boston University Stable Isotope Laboratory
- Center for Stable Isotope Biogeochemistry, UC Berkeley
- SIRFER, University of Utah
- Isotope ratio analysis at the Max Plank Institute for Biogeochemistry
(Please place a comment, beginning ‘ATTENTION EDITOR’, to the base of this page if you would like to add your lab to this list)
Ranges of values
Precipitation varies between -30 and +5‰ (VSMOW) around the world, with more depleted values at higher latitudes and altitudes. The Global Network of Isotopes in Precipitation is managed by the International Atomic Energy Agency (IAEA), and has been monitoring the hydrogen and oxygen content of precipitation at a large number of sites world-wide since 1961.
Leaf water is often enriched in 18O compared to soil water, and hence varies from -20 to +30‰ (VSMOW) around the world. Water vapour is depleted in 18O compared with precipitation or soil water. The IAEA MIBA (Moisture Isotopes in the Biosphere and Atmosphere) network measured the stable isotope composition of water pools in the terrestrial biosphere and the atmosphere (in this case, usually within-canopy air) at a number of sites around the world in 2005 and 2006.
Health, safety and hazardous waste disposal considerations
Please consult the relevant MSDS guides before using any of the chemicals listed in this summary
Related techniques
See associated PrometheusWiki pages:
- Isotope methods
- Structural compounds – Cellulose extraction
- 13C – Carbon isotopes and transpiration efficiency
- Deuterium and Hydrogen – Hydrogen isotopes
Literature references
Barbour MM, Farquhar GD (2000) Relative humidity- and ABA-induced variation in carbon and oxygen isotope ratios of cotton leaves. Plant, Cell & Environment 23, 473:485.
Bottinga Y, Craig H (1969) Oxygen isotope fractionation between CO2 and water, and the isotopic composition of marine atmospheric CO2. Earth and Planetary Science Letters 5, 285:295.
Cernusak LA, Pate JS, Farquhar GD (2002) Diurnal variation in the stable isotope composition of water and dry matter in fruiting Lupinus angustifolius under field conditions. Plant, Cell & Environment 25, 893:907.
Cernusak LA,Wong SC, Farquhar GD (2003) Oxygen isotope composition of phloem sap in relation to leaf water in Ricinus communis. Functional Plant Biology 30, 1059:1070.
Cuntz M., Ogée J.,Farquhar G.D., Peylin P.& Cernusak L.A. (2007) Modelling advection and diffusion of water isotopologues in leaves. Plant, Cell & Environment 30, 892:909.
Craig H, Gordon LI (1965) Deuterium and oxygen-18 variations in the ocean and the marine atmosphere. In ‘Proceedings of a conference on stable isotopes in oceanographic studies and paleotemperatures’. (Ed. E Tongiorgi) pp. 9:130. (Laboratory of Geology and Nuclear Science: Pisa)
Dongmann G, Nurnberg HE, Forstel H,WagenerK (1974) On the enrichment of H218O in the leaves of transpiring plants. Radiation and Environmental Biophysics 11, 41:52.
Farquhar GD, Lloyd J (1993) Carbon and oxygen isotope effects in the exchange of carbon dioxide between terrestrial plants and the atmosphere. In ‘Stable isotopes and plant carbon-water relations’. (Eds JR Ehleringer, AE Hall, GD Farquhar) pp. 47:70. (Academic Press: San Diego)
Helliker BR, Ehleringer JR (2000) Establishing a grassland signature in veins: 18O in the leaf water of C3 and C4 grasses. Proceedings of the National Academy of Sciences USA 97, 7894:7898.
Ogee J, Cuntz M, Peylin P, Bariac T (2007) Non-steady-state, non-uniform transpiration rate and leaf anatomy effects on the progressive stable isotope enrichment of leaf water along monocot leaves. Plant, Cell & Environment 30, 367-387.
Roden JS, Lin G, Ehleringer JR (2000) A mechanistic model for interpretation of hydrogen and oxygen isotope ratios in treering cellulose. Geochimica et Cosmochimica Acta 64, 21:35.
Sternberg L, DeNiro M (1983) Biogeochemical implications of the isotopic equilibrium fractionation factor between oxygen atoms of acetone and water. Geochimica et Cosmochimica Acta 47, 2271:2274.
Yakir D, DeNiro M, Gat J (1990) Natural deuterium and oxygen-18 enrichment in leaf water of cotton plants grown under wet and dry conditions: evidence for water compartmentation and its dynamics. Plant, Cell & Environment 13, 49:56.